Man Blames Injuries on Defective Zimmer M/L Taper Hip System
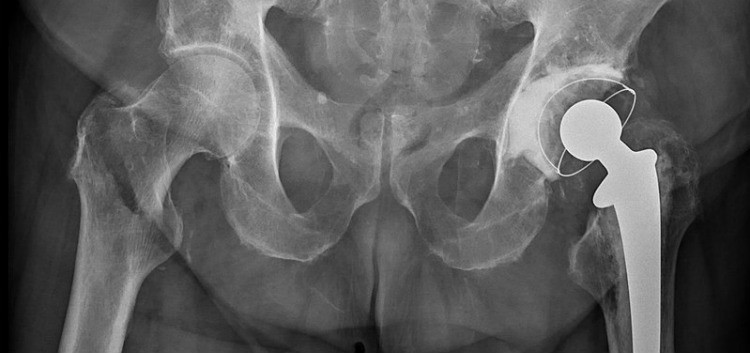
Robert Flick underwent two hip replacement surgeries. The first one was supposed to eliminate his pain and help with his hip issues. However, this implant turned into a nightmare. It gave him even more debilitating pain and other long-term complications.
His second surgery was necessary to correct his first one. After the second surgery, he sued the manufacturers of the device that he had implanted in his first surgery because, he claimed, it was directly to blame for his injuries and his total hip replacement failure.
The Surgeries
Robert Flick underwent hip replacement surgery in 2012. The device that was used was the Zimmer M/L Taper Hip System. After the surgery, his pain was supposed to decrease and he should have had more mobility.
The opposite happened and his pain increased. Doctors also noted that he had unsafe blood levels of chromium and cobalt. Finally, he was diagnosed with a complete hip replacement failure, synovial metallosis, and severe trunnionosis.
In 2016, he underwent a hip replacement revision surgery. This surgery was to remove the first implant and replace it with a second and different device. While in this surgery, doctors noted a massive solid pseudotumor with necrosis and grade two trunnion corrosion or the implant device.
The Device
The Zimmer M/L Taper Hip System contains the following four parts:
- The stem made of titanium alloy,
- The head made of chromium and cobalt,
- The shell made of titanium alloy, and
- A liner made of polyethylene.
The implant is marketed by the manufacturer as a metal-on-polyethylene device. This is supposed to decrease the risk of corrosion and failure of the surgery.
However, the Zimmer M/L Taper Hip System is not completely a metal-on-polyethylene product. The metal stem is connected to the metal head, which makes the product a partial metal-on-metal device. These types of devices are more corrosive and known for higher failure rates.
The Lawsuit
Robert Flick, the Plaintiff in the case, sued the manufacturer of the Zimmer M/L Taper Hip System. He claimed that the manufacturer, Zimmer, was strictly liable for his injuries. Strict product liability is when a manufacturer is liable for a defective product. This is regardless of whether the plaintiff in the lawsuit had any fault in the injury. There are three types of strict product liability: warning defect, design defect, and manufacturing defect.
A warning defect is when a manufacturer or company did not provide adequate warnings or instructions on how to use the product. An injury occurs because of the lack of warning or instructions.
A design defect is when the actual initial and used design of a product makes the use of the product unsafe. A manufacturing defect is when the design was not unsafe but there was an issue with the manufacturing process that made the product unsafe.
Here, the Plaintiff claims that the manufacturer is strictly liable because it should have known that metal-on-metal devices were more corrosive. The manufacturer failed to warn doctors and patients about these potential risks.
The manufacturer is also strictly liable, according to the Plaintiff, because the design was unsafe. A simple ceramic head in the device would eliminate the metal-on-metal corrosive risk.
Finally, the Plaintiff claims strict liability because the device was manufactured in a way that deviates from the normal way hip devices are made by other manufacturers.
Additional Information
Robert Flick also claimed the manufacturers were guilty of negligence and negligent misrepresentation because they did not exercise ordinary and reasonable care in the design, manufacture, testing, quality assurance, marketing, and warning of the device. The manufacturer should have known that a metal-on-metal device could cause these injuries and they failed to take action.
Additionally, the lawsuit claimed the manufacturers breached both an express and implied warranty. This is because it was marketed specifically to treat the condition that the Plaintiff had, however, it was clearly not meant to do so. The device also did not meet the expectations for its use and performance because it was expressly the cause of the man’s injuries.
One other main issue that will be addressed in this court case is the U.S. Food and Drug Administration (FDA) 501(k) clearance procedure. This is a fast-track approval system that allows a product to go on the market before it is fully tested. The manufacturer only needs to show that its product is similar to others on the market.
In this case, Zimmer was able to get 501(k) clearance on its Zimmer M/L Taper Hip System. This means that the product did not go through a rigorous testing process before it was put on the market and used by patients like Robert Flick.
